- Startseite
- Infrastruktur
- Labor-Infrastrukturen
- Gas-Isotopenverhältnis-Massenspektrometer
- Mass Spectrometry on Inorganic Samples
- Guidelines and Terms of Use (Clumped Isotopes)
Guidelines and Terms of Use (Clumped Isotopes)
NOTE
Measurements of Clumped Isotopes are in the experimental phase. These Guidelines and Terms of Use are preliminary, and are subject to frequent changes as methods and analytical procedures develop.
Version: 04 May 2022
Responsible person: Henning Kuhnert
Contents
Introduction
This document complies with the requirements for the "Nutzungsordnung" according to DFG form 55.04, 06.2016 – "Hinweise zu Gerätenutzungskosten und zu Gerätezentren".
The guidelines and terms of use in this document are generally binding for all users.
Clumped Isotope analyses are carried out in collaboration with the working group "Paleoceanography". New users will be jointly introduced to the analytical and data handling techniques, and the laboratory safety procedures.
Persons entitled to use the laboratory
The MARUM Stable Isotope Laboratory accepts samples from MARUM scientists. Within capacity limits, the laboratory accepts samples from other scientists at the University of Bremen within non-commercial projects. Persons from outside these groups are accepted as users at the discretion of the head of the laboratory, the head of the Paleoceanography working group and the MARUM director.
Users are required to possess documented basic knowledge of and practical skills in carrying out work in chemical laboratories and handling of hazardous materials. This includes, for example, successful participation in practical laboratory courses for students.
Acknowledgment in publications
When publishing data, the laboratory must be acknowledged as "MARUM Stable Isotope Laboratory, University of Bremen" or equivalent statements, typically in the "Materials and Methods" section of a journal article.
For describing the methods, a template is provided in the Appendix. Please, check the measurement data report to make sure you provide the correct analytical details. Copying the text from previous publications will more often than not result in incorrect statements.
Contact
For technical and scientific questions, please contact the head of the laboratory (see below) or the clumped isotope specialists of the Paleoceanography working group.
How much does it cost?
MARUM and University of Bremen
The in-house price for a full tray (46 positions, including standards) is €180, and proportionally less for incompletely filled trays. Note that measurement of one sample requires 20-30 positions (replicates), not accounting for the necessary standards.
After receipt of the analyses results the user will be asked to provide the account details ("Fondsnummer"). Usually, no further action is required on part of the user.
External collaborators
For Clumped Isotope analyses, users are required to carry out some of the laboratory work themselves, and hence no external samples are accepted.
Commissioned work
For Clumped Isotope analyses, we do not carry out commissioned (commercial) work.
Order of access through projects and users
Projects and users receive instrument access in the order of incoming requests, but projects of the Paleoceanography working group and MARUM take precedence. In the event of a severe backlog, the laboratory may ask users to change the order of measurements so that urgent project deadlines can be met (end of bachelor and master projects; end of funding period; report for funding agency; …).
Hints for planning
When planning your project, please keep in mind the limited sample throughput, and contact the laboratory in advance.
Samples and standards must be weighed and loaded into the sample vial, ideally on the day before running the mass spectrometer. While not difficult, weighing microsamples is very time-consuming, requires patience and takes several hours for a full tray. Plan ahead to avoid pressure of time (a good idea for laboratory work in general).
What types of samples are accepted?
- Foraminifera and ostracods (whole shells or crushed).
- Bulk sediment. The carbonate content should exceed 30%, and the sediment must be ground down to silt grain size (but no dust, if possible).
- Samples retrieved through micro-drilling. This includes (among others) corals, molluscs, otoliths, and many abiogenic carbonates (e.g., speleothems).
- Material from filter membranes. This includes coccolithophores and calcareous dinoflagellates from culture experiments and ship cruises. The membranes must consist of polycarbonate.
Other material: Contact us.
Samples containing significant amounts of hydrocarbons are not accepted.
All samples must be dry.
How much material is required?
For each replicate, 110 μg are required, or up to 3 mg per sample. As a guideline, a single planctonic foraminifer shell within the size fraction 250-350 µm weighs 10 µg.
For ground or powder samples +1 mg should be available, as material clings to the wall of the sample vial and cannot be retrieved.
Are specific cleaning and preparation procedures required?
Clumped isotopes are very sensitive to diagenetic alterations (recrystallization of carbonates and cementation). Users should carefully screen their samples and remove contaminant carbonates, if present.
Removal of non-carbonate phases is necessary only if these are volatile in vacuum or release gases when exposed to phosphoric acid. Most normal rock constituents, including clay minerals, are unproblematic.
Foraminifer samples should be cleaned following the "Mg/Ca protocol" (Barker et al., 2003), i.e., including multiple ultrapure water and methanol rinses, ultrasonication steps and oxidative cleaning.
Important note
Never use antistatic wipes on the samples or their containers. Never use vials or tools containing silicones (polysiloxanes), such as lids with silicone rubber seals or metal tools covered in silicone oil as lubricant. They release chemicals that become volatile under vacuum and contaminate the mass spectrometer by accumulating in the ion source and the flight tube. The instrument then needs very time-consuming and expensive servicing.
How do I store my samples?
Use transparent centrifuge tubes or microbeakers of 1.5 mL volume (or larger). Containers must be placed in trays. See Figure 1.
Sample labels
All samples must be properly labeled. Abbreviated identifiers are fine as long as they remain unambiguous and clearly correspond to the accompanying documentation (see below). When using adhesive labels on beakers or tubes we recommend thin plastic labels, or paper labels that are secured with clear tape. Normal paper labels are a bit stiff and have the tendency to detach at the ends. Writing directly on the container is an alternative, of course.
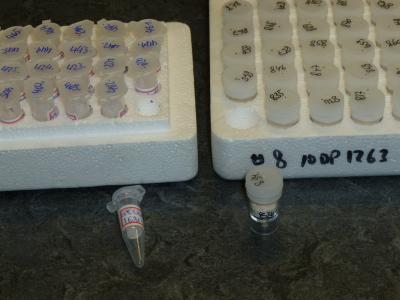
How do I document my samples?
Samples must be accompanied by a tabular sample list in both printed and digital form. The samples must appear on the list in the same order as on the tray or in the box. Please, use the following table format:
Header: Name, email, and affiliation of submitter; type of samples (e.g., bulk sediment), region or name of site / outcrop.
Columns: Site, sample depth or running number, species (for forams), number of specimens (for forams), % carbonate content (for bulk rock).
Please, do not use the cryptic IODP and MeBo sample identifiers. Identifiers are typed in manually in the spectrometer software, and these excessively complicated formats are prone to errors.
Each line in the list must correspond to a separate sample container and vice versa.
An Excel template is available upon request.
How is the measurement carried out?
Clumped Isotopes are measured on a ThermoFisher 253plus mass spectrometer coupled to a Kiel IV carbonate device. In addition to the usual detectors for masses 44, 45, 46 the spectrometer has additonal detectors for masses 46.5, 47, 48 and 49, and the carbonate device is equipped with a cold trap for hydrocarbon removal. In the carbonate device the sample reacts with phosphoric acid at 70 °C under vacuum. The evolving CO2 gas is dried and transferred to the mass spectrometer via a dual inlet system. For each sample, the dual inlet switches between the sample CO2 and a reference CO2; the instrument software calculates the sample composition from the comparison of both gases. A total of eight paired sample-reference measurements is performed and their mean value is reported. The mass spectrometer does not directly measure carbon and oxygen isotopes, but isotopologues of CO2: 12C16O16O (mass 44), 13C16O16O (mass 45), 12C16O18O (mass 46), and 13C16O18O (mass 47). δ13C is derived from the intensity ratios for masses 45 and 44, δ18O from masses 46 and 44, and δ47 from masses 47 and 44. All isotopic ratios are determined simultaneously.
How are the data evaluated?
Each sequence includes multiple measurements of four different types of carbonate reference standards. Currently, we use ETH I-IV standards kindly provided by S. Bernasconi, ETH Zürich. Appropriate house standards will be generated once the analytical setup has been finalized.
Raw measurement data are evaluated with the Easotope software that calibrates the sample data against the standard measurements.
What repeatability is achieved?
The repeatability is the standard deviation for the measurements of the carbonate standards (see above). Typical values will be reported once sufficient long-term information is available. Specific values for each set of measurements come along with the respective measurement data.
How are measurement data documented and archived?
Public information
Upon publishing their data, typically in the form of peer-reviewed journal articles, users are obliged to make their data publicly available through the PANGAEA data base.
Data storage
Digital copies of each daily run and each sample series and a printout of the first are kept by the laboratory. Evaluated data are kept on the server for the Easotope software by the Paleoceanography working group. Data are not passed to third parties.
Appendix: Template for "Materials and Methods"
Users may adapt the following example for describing the methods for measuring Clumped Isotopes.
"Samples were measured at MARUM, Center for Marine Environmental Sciences, University of Bremen, on a ThermoFisher 253plus gas isotope ratio mass spectrometer connected to a Kiel IV automated carbonate preparation device. The latter was equipped with a custom-made Porapak cold trap operated at -30 °C for hydrocarbon removal. Data are reported in the usual delta- and delta-47-notations versus V-PDB. The instrument was calibrated against the ETH I-IV standards (Bernasconi et al., 2018). Data evaluation was carried out using the Easotope software (www.easotope.org)."
This text is preliminary and subject to changes.


