- Startseite
- Infrastruktur
- Labor-Infrastrukturen
- Gas-Isotopenverhältnis-Massenspektrometer
- Mass Spectrometry on Inorganic Samples
- Guidelines and Terms of Use (Carbonates)
Guidelines and Terms of Use (Carbonates)
Version: 23 May 2022
Responsible for the content: Henning Kuhnert
Contents
Introduction
This document complies with the requirements for the "Nutzungsordnung" according to DFG form 55.04, 06.2016 – "Hinweise zu Gerätenutzungskosten und zu Gerätezentren".
The guidelines and terms of use in this document are generally binding for all users.
The MARUM Stable Isotope Laboratory operates as a service facility, where users submit their samples, but do not carry out laboratory work themselves, unless the user and the head of the laboratory have mutually agreed otherwise. Users are primarily "sample submitters". It is their responsibility to meet the requirements for the samples as outlined in this document. The laboratory staff carries out the laboratory work proper, namely the analyses of stable carbon (δ13C) and oxygen (δ18O) isotope ratios of carbonate material.
Persons entitled to use the laboratory
The MARUM Stable Isotope Laboratory accepts samples from scientists of MARUM and the Department of Geosciences, University of Bremen. Within capacity limits, the laboratory accepts samples from other scientists at the University of Bremen within collaborative projects with MARUM. Persons from outside these groups are accepted as users at the discretion of the head of the laboratory and the MARUM director.
Acknowledgment in publications
When publishing data, the laboratory must be acknowledged as "MARUM Stable Isotope Laboratory, University of Bremen" or equivalent statements, typically in the "Materials and Methods" section of a journal article. Additional rules might apply within cooperation agreements.
For describing the methods, a template is provided in the appendix. Please, check the measurement data report to make sure you provide the correct instrument type and standard deviation. Copying the text from previous publications will more often than not result in incorrect statements.
Contact
For technical and scientific questions, please contact the head of the laboratory:
How much does it cost?
MARUM and University of Bremen
The in-house price of €5 per sample is charged to cover the consumables. After receipt of the analyses results the user will be asked to provide the account details ("Fondsnummer"). Usually, no further action is required on part of the user.
External collaborators
The price for measurements within collaborative research projects with external institutions, where the latter cover the costs, is €6.25 per sample (including 20% administrative overhead). The collaboration usually requires that a MARUM scientist is co-applicant in the respective project. A non-formal cooperation with the intention to include the measurement data in a joint publication is a collaboration by common sense, but not by EU law.
Commissioned work
The price for commercial measurements is about €16 per sample (plus VAT). For legal reasons, this is a non-binding estimate that does not represent a quotation. The latter is available upon request. Commercial customers have the intellectual ownership of the measurement data.
How long will it take to obtain the data?
Normally, the turnaround time is on the order of one to four months, depending on the number of new samples submitted and those already in the line. The daily throughput per machine is 36 samples.
Is there a way to prioritize samples?
The samples are processed on a first come, first served basis. Exception: Samples from University of Bremen bachelor and master projects will be given priority, but the number of samples is limited to about 150 per project.
In the event of a severe backlog, the laboratory might change the order of measurements so that users can meet urgent project deadlines (end of bachelor and master projects; end of funding period; report for funding agency; …). Internal samples take precedence over external samples in this case.
What types of samples are accepted?
- Foraminifera and ostracods (whole shells or crushed).
- Bulk sediment. The carbonate content should exceed 30% (and must be documented where it is less than 70%), and the sediment must be ground down to silt grain size (but no dust, if possible).
- Samples retrieved through micro-drilling. This includes (among others) corals, molluscs, otoliths, and many abiogenic carbonates (e.g., speleothems).
- Material from filter membranes. This includes coccolithophores and calcareous dinoflagellates from culture experiments and ship cruises. The membranes must consist of polycarbonate.
Other material: Contact us.
Samples containing significant amounts of hydrocarbons are not accepted.
All samples must be dry.
How much material is required?
The minimum amount is 20-40 µg carbonate (depending on the spectrometer), but >150 µg are preferred, as this allows repeat measurements, should the need arise. As a guideline, a single planctonic foraminifer shell within the size fraction 250-350 µm weighs 10 µg.
For ground or powder samples at least 1 mg is required, as the material clings to the wall of the sample vial. There is no upper limit to the sample weight upon submission, the laboratory staff takes care of using the right amount.
Are specific cleaning and preparation procedures required?
Samples are measured in the shape in that they arrive. Foraminifera should be thoroughly washed before submission to remove micrite fillings from chambers; usually this is already accomplished during wet sieving.
Removal of contaminant phases is necessary only if these are volatile in vacuum or release gases when exposed to phosphoric acid. Most normal rock constituents, including clay minerals, are unproblematic.
Submission of pre-treated samples
The laboratory has to be informed on treatments with organic chemicals before sample submission. Ethanol has a molecular mass of 46 and interferes with the determination of δ18O. Also avoid using glue (e.g., for holding forams in place). Treatment with hydrogen peroxide (H2O2) is not critical.
Important note
Never use antistatic wipes on the samples or their containers. Never use vials or tools containing silicones (polysiloxanes), such as lids with silicone rubber seals or metal tools covered in silicone oil as lubricant. They release chemicals that become volatile under vacuum and contaminate the mass spectrometer by accumulating in the ion source and the flight tube. The instrument then needs very time-consuming and expensive servicing.
How do I store my samples?
Internal samples: Foraminifera and Ostracods
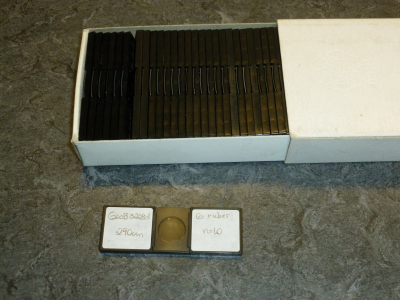
Plastic microslides with single holes and plastic lids are preferred (Fig. 1). Please, do not submit dual- or multi-hole slides, they are difficult for us to handle. The slides must be stored in fitting boxes or trays. Avoid cardboard slides with glass lids; the lids break easily, and the cardboard gives off particles.
Internal samples: All other samples
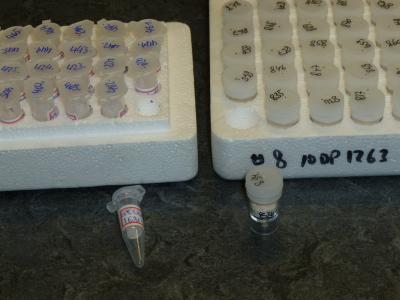
Please, use transparent centrifuge tubes of 1.5 mL volume, or micro-beakers with cone-shaped bottom, also 1.5 mL (Fig. 2). Do not submit smaller containers; it is difficult retrieving defined sample amounts from these. The vials must be stored in trays.
External samples sent by mail
Use 1.5 mL transparent centrifuge tubes for all types of samples. For foraminifera, single-hole plastic microslides may alternatively be used (see above). Please, do not fix the lids of the sample containers with tape or parafilm, this generates additional and unnecessary work for the laboratory staff. Containers must be placed in trays. If the tray has no lid, kitchen cling film may be used to prevent the vials from falling out (Fig. 3).
Sample labels
All samples must be properly labeled. Abbreviated identifiers are fine as long as they remain unambiguous and clearly correspond to the accompanying documentation (see below). When using adhesive labels on beakers or tubes we recommend thin plastic labels, or paper labels that are secured with clear tape. Normal paper labels are a bit stiff and have the tendency to detach at the ends. Writing directly on the container is an alternative, of course.

How do I document my samples?
Samples must be accompanied by a tabular sample list in both printed and digital form. The samples must appear on the list in the same order as on the tray or in the box. Please, use the following table format:
Header: Name, email, and affiliation of submitter; type of samples (e.g., bulk sediment), region or name of site / outcrop.
Columns: Site, sample depth or running number, species (for forams), number of specimens (for forams), % carbonate content (for bulk rock).
Please, do not use the cryptic IODP and MeBo sample identifiers. Identifiers are typed in manually in the spectrometer software, and these excessively complicated formats are prone to errors and longer than the software can handle.
Each line in the list must correspond to a separate sample container and vice versa.
An Excel template is available upon request.
Where do I submit my samples?
When your samples are properly stored, labeled, and documented... Marum: Just bring them along and pass them to:
Henning Kuhnert, MARUM I 1150, Phone 65520; or Birgit Meyer, MARUM I 1190, Phone 65524.
External samples shipped by mail should be addressed to:
Henning Kuhnert
Universität Bremen
MARUM I, 1150
Leobener Straße 8
28359 Bremen
Germany
Customs declaration
Shipments from inside the EU do not require customs documents. Otherwise, check details with your shipping company. A common pitfall is declaring scientific samples as "samples". In customs terms this refers to ware samples (such as trial packages) which makes the shipment subject to taxes. Use "rock samples" instead. Provide a value between 10 and 20€ for the total content. A value of 0 is not accepted by the customs (why would you pay shipping costs for something worthless?). A value exceeding 20€ makes the shipment subject to import taxes. Should these additional costs arise, the laboratory will charge them to the sample submitter.
How is the measurement carried out?
We currently operate three systems, a Finnigan MAT 251 gas isotope ratio mass spectrometer coupled to a Kiel I automated carbonate preparation device, a Finnigan MAT 252 coupled to a Kiel III, and a ThermoFisher 253plus coupled to a Kiel IV. In the carbonate device the sample reacts with phosphoric acid at 70-75 °C under vacuum. The evolving CO2 gas is dried and transferred to the mass spectrometer via a dual inlet system. For each sample, the dual inlet switches between the sample CO2 and a reference CO2; the instrument software calculates the sample composition from the comparison of both gases. A total of eight paired sample-reference measurements is performed and their mean value is reported. The mass spectrometer does not directly measure carbon and oxygen isotopes, but isotopologues of CO2: 12C16O16O (mass 44), 13C16O16O (mass 45), and 12C16O18O (mass 46). δ13C is derived from the intensity ratios for masses 45 and 44, δ18O from the intensity ratios for masses 46 and 44. δ13C and δ18O are determined simultaneously.
What type of material is used for calibration?
Each sequence of 36 samples additionally includes 9 positions for the house standard. This house standard consists of ground and sieved Solnhofen limestone of known isotopic composition and is used for instrument calibration. The house standard was itself calibrated against the NBS 19.
Note that the calibration is always against calcite; data from the measurement of other carbonate minerals need to be converted by the user based on the different phosphoric acid fractionation factors for δ18O. Examples for aragonite and dolomite are available upon request. No conversion is required for δ13C.
What repeatability is achieved?
The repeatability is the standard deviation for the measurements of the house standard (see above). Typical values are 0.04‰ for δ13C and 0.06‰ for δ18O. The exact numbers are provided with each set of measurements.
How are measurement data documented and archived?
Public information
Upon publishing their data, typically in the form of peer-reviewed journal articles, non-commercial users are obliged to make their data publicly available through the PANGAEA data base.
User information
The sample submitter receives an Excel workbook with two spreadsheets via email. Each line of the first spreadsheet contains the sample name, the raw data (δ13C, δ18O as reported by the instrument software), the calibrated data (offline-corrected for the measurements of the laboratory standard), and the internal standard deviation. Each measurement is associated with a unique identifier, consisting of an identifier for the mass spectrometer and a running number. Process information for each measurement includes the signal intensities and pressures of the sample and reference gases, the number of expansions, and the measurement date. Additional process information depends on the instrument.
A separate column of the spreadsheet reports series-specific information, including the unique identifier of the sample series (a running number), the type of instrument, and the repeatability of the laboratory standard.
The second spreadsheet contains the measurement information of the laboratory standards that were used for calibration.
Data storage
Digital copies of each daily run and each sample series and a printout of the first are kept by the laboratory. Data are not passed to third parties.
Appendix: Template for "Materials and Methods"
Users may adapt the following example for describing the methods for measuring δ13C and δ18O.
"Samples were measured at MARUM, Center for Marine Environmental Sciences, University of Bremen, on a [1] gas isotope ratio mass spectrometer connected to a Kiel [1] automated carbonate preparation device. Data are reported in the usual delta-notation versus V-PDB. The instrument was calibrated against the house standard (ground Solnhofen limestone), which in turn was calibrated against the NBS 19 calcite. Over the measurement period the standard deviations of the house standard were [2]‰ for δ13C and [2]‰ for δ18O."
For non-calcite samples, also provide the reaction temperature (75 °C). As the measurements are always calibrated against calcite, users are required to carry out any corrections for the phosphoric acid fractionation factors of other carbonate minerals themselves.
Information in [] is provided in the upper right on the first spreadsheet of the data workbook.
[1] Valid pairs include:
Finnigan MAT 251 and Kiel I
Finnigan MAT 252 and Kiel III
ThermoFisher 253plus and Kiel IV.
[2] These values are the standard deviations of the carbonate standard data in the second spreadsheet.


