Page path:
- General/Marine Geology
- Gas Hydrate Research
- Struktur und Stabilität
Struktur und Stabilität
Gas hydrates are non-stoichiometric compounds. Water molecules (so-called structural molecules) form cage-like structures in which gas molecules are enclosed as guest molecules. For this reason, they are also called cage compounds or clathrates (lat.: clathratus = cage). Generally speaking, gas hydrate can contain different types of gas molecules in separate cages, depending on the mixture of gas molecules in the direct environment. In addition to CH4 (methane) and C2H6 (ethane) in naturally occurring gas hydrates these gases will mostly be H2S, CO2 and, less frequently, other hydrocarbons.
Picture:
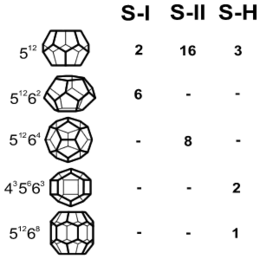
Three different structures of gas hydrate are known so far: I, II and III, which are built of various numbers of the five cage types shown here. The simplest cage type is the pentagonal dodecahedron (512). It is found in all three structures, whereas the other cage types are only found in one of the structures.
Three different structures of gas hydrate are known so far: I, II and III, which are built of various numbers of the five cage types shown here. The simplest cage type is the pentagonal dodecahedron (512). It is found in all three structures, whereas the other cage types are only found in one of the structures.
To date gas hydrates of three different crystal structures have been identified. Structures sI and sII both crystallize to a cubic (isometric) system, whereas the third structure (denominated H) crystallizes to a hexagonal system, like ice. The gas hydrate structure can be seen as a packing of polyhedral cages. All three structures occur in nature with structure I being most frequent. Its unit cell consists of 8 cages of two different types which can enclose gas molecules that are smaller in diameter than propane molecules, such as CH4, CO2 or H2S. Due to the specifics in gas composition, the natural occurrence of sI hydrates mainly depends on the presence of biogenic gas, as commonly found in marine sediments.
A unit cell of structure II consists of 24 cages, i.e. 16 small cages and 8 large ones. The latter are larger than those of structure I. Hence, structure II contains natural mixtures of gases with molecules larger than ethane and smaller than pentane. It is usually confined to areas where thermogenic formation of gas in relatively deep sediments takes place. Structure H is a more complex structure. Apart from smaller cages, it contains a cage type which requires very large gas molecules such as methyl cyclohexane.
A unit cell of structure II consists of 24 cages, i.e. 16 small cages and 8 large ones. The latter are larger than those of structure I. Hence, structure II contains natural mixtures of gases with molecules larger than ethane and smaller than pentane. It is usually confined to areas where thermogenic formation of gas in relatively deep sediments takes place. Structure H is a more complex structure. Apart from smaller cages, it contains a cage type which requires very large gas molecules such as methyl cyclohexane.

Picture:
A melting lump of methane hydrate. As the hydrate dissociates, methane is released to feed a constant flame ("burning ice") while the escaping water drops off.
A melting lump of methane hydrate. As the hydrate dissociates, methane is released to feed a constant flame ("burning ice") while the escaping water drops off.
Gas hydrates only form at high pressure and a comparatively low temperature and if sufficient amounts of gas and water are present. H2S, CO2 and higher hydrocarbons shift the hydrate-gas phase boundary to higher temperatures, whereas nitrogen and dissolved salts shift it to lower temperatures.


