Die Inhalte dieser Seite sind leider nicht auf Deutsch verfügbar.
Seitenpfad:
- Organische Geochemie
- Forschung
- Biomarkers
Hinrichs Lab - Biomarkers
Introduction
Biomarkers are organic molecules that are found in modern or ancient environments and can be traced back to a biological origin.
Biomarkers were first identified by the German chemist Alfred Treibs (1899-1983). In 1936 Treibs reported the occurrence of chlorophyll and other porphyrin-derived breakdown products in oils, shales and coals around the world. He interpreted the presence of these plant-derived fossil molecules as an indicator for the biological origin of petroleum. With his studies, Treibs laid the foundation of modern petroleum geochemistry.
Following the work of Treibs, more scientists began to apply their expertise in organic chemistry to geologic materials. This led to the identification of novel biomarkers in environments ranging from sediments to ancient rocks and oils. The discovery of new biomarkers was accompanied by thorough analyses of their biological sources and of their dependence on environmental conditions.
The application of biomarkers in microbial ecology and micropalaeontology requires the integration of disciplines such as organic geochemistry, microbiology and inorganic geochemistry. In the Hinrichs Lab we use interdisciplinary approaches to understand biomarker patterns in modern and ancient environments using state-of-the-art analytical techniques.
Biomarkers were first identified by the German chemist Alfred Treibs (1899-1983). In 1936 Treibs reported the occurrence of chlorophyll and other porphyrin-derived breakdown products in oils, shales and coals around the world. He interpreted the presence of these plant-derived fossil molecules as an indicator for the biological origin of petroleum. With his studies, Treibs laid the foundation of modern petroleum geochemistry.
Following the work of Treibs, more scientists began to apply their expertise in organic chemistry to geologic materials. This led to the identification of novel biomarkers in environments ranging from sediments to ancient rocks and oils. The discovery of new biomarkers was accompanied by thorough analyses of their biological sources and of their dependence on environmental conditions.
The application of biomarkers in microbial ecology and micropalaeontology requires the integration of disciplines such as organic geochemistry, microbiology and inorganic geochemistry. In the Hinrichs Lab we use interdisciplinary approaches to understand biomarker patterns in modern and ancient environments using state-of-the-art analytical techniques.

Our research approach
We decipher information from various types of organic molecules which are biomarkers in a broad sense. These molecules include (1) high-molecular-weight compounds that form important building blocks of microbial cells, carry taxonomic information, and record proxy information on growth conditions, (2) low-molecular-weight compounds that act as central intermediates in metabolic processes, amd (3) dissolved organic matter (DOM) in sediment pore-waters that potentially links the pool of recalcitrant macromolecular organic matter in sediment deposits to the pool of low-molecular-weight substrates available to microorganisms. While we seek to decode information from the structural and stable isotopic properties of individual compounds, the consideration of samples in their geoecological context as well as the advancement of analytical techniques are crucial parts of our work.
We use the natural abundance of stable carbon and hydrogen isotopes in pristine environmental samples to study how microbial communities, metabolic processes, and the global carbon cycle are linked to each other and we employ stable carbon isotope probing (SIP) to test our hypotheses on carbon flow in incubation experiments. Stable isotopic investigations of biomarkers in pristine samples are particularly useful in systems where cultivation based methods are at the limit of their applicability, for example in the energy-limited, metabolically slow deep biosphere. Here, the stable isotopic composition of membrane lipids and metabolites in sediments and sediment pore-waters, respectively, carries information that integrates microbial processes over long periods of time. Other examples for the use of biomarkers in our research are given below.
We study the taxonomic composition and population density of microbial communities, their metabolic processes and relation to geochemical context and global carbon cycle based on the information encoded in the qualitative, quantitative and isotopic composition of a broad range of organic compounds. Our research covers marine and terrestrial, past and present environments. This research approach requires continuous development and innovation of analytical methods.
We use the natural abundance of stable carbon and hydrogen isotopes in pristine environmental samples to study how microbial communities, metabolic processes, and the global carbon cycle are linked to each other and we employ stable carbon isotope probing (SIP) to test our hypotheses on carbon flow in incubation experiments. Stable isotopic investigations of biomarkers in pristine samples are particularly useful in systems where cultivation based methods are at the limit of their applicability, for example in the energy-limited, metabolically slow deep biosphere. Here, the stable isotopic composition of membrane lipids and metabolites in sediments and sediment pore-waters, respectively, carries information that integrates microbial processes over long periods of time. Other examples for the use of biomarkers in our research are given below.
We study the taxonomic composition and population density of microbial communities, their metabolic processes and relation to geochemical context and global carbon cycle based on the information encoded in the qualitative, quantitative and isotopic composition of a broad range of organic compounds. Our research covers marine and terrestrial, past and present environments. This research approach requires continuous development and innovation of analytical methods.

High-molecular-weight compounds: membrane lipids
Lipids are an essential building block of cell membranes. Typically, membrane-forming lipids consist of a hydrophilic polar head group and a hydrophobic tail, a structural property that leads to the arrangement of a lipid bilayer which separates the inside of a cell from the outside environment. Membrane lipids work not only as permeability barrier but are also involved in the transport of nutrients into the cell, signal transduction, and maintenance of the proton-motive force. After cell death, the biological membrane is easily damaged and the labile polar head groups of the IPLs can be rapidly hydrolyzed by enzymatic reactions.
With respect to biomarkers, we distinguish intact polar lipids (IPLs) from core lipids. IPLs are the genuine building blocks of cell membranes. They are not stable and are thought to decay rapidly after cell death. Based on this assumption, IPLs can be used to study modern, living organisms in remote systems like the deep biosphere. In contrast, core lipids are the stable apolar subunits of IPLs that survive as “molecular fossils”, in some cases for millions of years. They carry information that can be used for the reconstruction of paleo-environments. Decay of IPLs after cell death and mixing of both the modern IPL and ancient core lipid pools are subjects of ongoing research in the Hinrichs Lab.
With respect to biomarkers, we distinguish intact polar lipids (IPLs) from core lipids. IPLs are the genuine building blocks of cell membranes. They are not stable and are thought to decay rapidly after cell death. Based on this assumption, IPLs can be used to study modern, living organisms in remote systems like the deep biosphere. In contrast, core lipids are the stable apolar subunits of IPLs that survive as “molecular fossils”, in some cases for millions of years. They carry information that can be used for the reconstruction of paleo-environments. Decay of IPLs after cell death and mixing of both the modern IPL and ancient core lipid pools are subjects of ongoing research in the Hinrichs Lab.
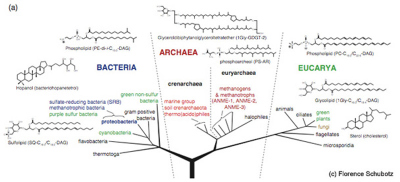
Phylogenetic tree of life after Woese et al. 1990 with representative membrane lipids of the three domains of life: Bacteria, Archaea and Eukarya (modified after Schubotz, 2009)
Evolutionary adaptations have led to the formation of a large diversity of membrane lipids with variations in both the hydrophilic polar head group and the hydrophobic tail or core lipid. As a results, membrane lipids carry taxonomic information. Phosphate- and glycosidic-based head groups are most common and form phospho- and glycolipids, respectively, in all three domains of life. However, Bacteria and Eukarya are very distinct from Archaea with respect to their apolar core lipids. The core lipids of Bacteria and Eukarya typically consist of fatty acid esters while isoprenoidal ethers are characteristic for Archaea. In the composition of core lipids, hopanoids are a distinct feature of Bacteria while steroids are a special characteristic of Eukarya. The carbon chain length of the fatty acids as well as the number of unsaturations or positions of methylations can be specific for certain types of Bacteria or Eukarya. Also, Archaea can have varying carbon chain lengths in their ether lipids and contain either tetraether or diether lipids with hydroxyl groups or different degrees of cyclization. The taxonomic information in lipid biomarkers allows us to distinguish different groups of organisms but is not as specific as the genetic information that is encoded on small subunits of the ribosomal RNA (16S rRNA) and suitable for the differentiation between single species.
Moreover, the chemical structures of membrane lipids are also related to environmental conditions. For example, changes in lipid composition help to adapt membrane fluidity to external variations in pressure, temperature, and pH. Due to their systematic response to environmental conditions, membrane lipids can be used as proxies for the environmental conditions under which the organisms grew. This is particularly true for the stable core lipids that survive as molecular fossils and can be used for the reconstruction of paleo-environments. Classical applications include the determination of past sea surface and ocean temperatures by using the paleo-proxies UK’37 and TEX86 that are based on the relative distribution of unsaturation of the C37 alkenones (e.g., Prahl & Wakeham, 1987) or the internal variation of pentacyclic rings of glycerol dialkyl glycerol tetraethers (GDGTs; Schouten et al., 2002), respectively.
Among the building blocks of microbial cells, membrane lipids are particularly interesting as they provide a unique view of natural microbial ecosystems. They carry information on the phylogenetic composition of the ecosystem, the population density, and the physicochemical and physiological conditions under which microbial community members existed and on their carbon sources, not only in modern but also in ancient environments.

Low-molecular-weight compounds: metabolites
We study various low-molecular-weight compounds that act as central intermediates in metabolic processes, including volatile fatty acids (VFAs), volatile organic sulfur compounds, and methane (CH4).
Volatile fatty acids (VFAs) are key compounds in anaerobic metabolism and cycling of carbon in marine sediments. For example, the water-soluble C2-compound acetate is produced by fermentation of organic matter as well as by acetogenesis (i.e. the reduction of inorganic CO2 along the acetyl-CoA pathway) and it serves as an important substrate for a variety of microorganisms including sulfate reducing bacteria and methanogens.
Methylated sulphides like dimethyl sulfide (DMS) and methanethiol (MT) are the most abundant volatile organic sulfur compounds. In anoxic environments, DMS and MT link carbon and sulfur cycles in diverse ways. While many biogeochemical processes involving methylated sulfides have been discovered during laboratory incubation, characterizing the distribution of these compounds in anoxic sediments remains a great analytical challenge.
Methane and other volatile hydrocarbons (C1 to C6) are formed during microbial and/or thermal degradation of organic matter. Using stable isotope analysis, we have studied their sources and sinks (e.g. Hinrichs et al. 1991, 2006). The formation and consumption of methane is mediated by archaea and the involved processes and carbon flow are a focus of our research activitites.
Volatile fatty acids (VFAs) are key compounds in anaerobic metabolism and cycling of carbon in marine sediments. For example, the water-soluble C2-compound acetate is produced by fermentation of organic matter as well as by acetogenesis (i.e. the reduction of inorganic CO2 along the acetyl-CoA pathway) and it serves as an important substrate for a variety of microorganisms including sulfate reducing bacteria and methanogens.
Methylated sulphides like dimethyl sulfide (DMS) and methanethiol (MT) are the most abundant volatile organic sulfur compounds. In anoxic environments, DMS and MT link carbon and sulfur cycles in diverse ways. While many biogeochemical processes involving methylated sulfides have been discovered during laboratory incubation, characterizing the distribution of these compounds in anoxic sediments remains a great analytical challenge.
Methane and other volatile hydrocarbons (C1 to C6) are formed during microbial and/or thermal degradation of organic matter. Using stable isotope analysis, we have studied their sources and sinks (e.g. Hinrichs et al. 1991, 2006). The formation and consumption of methane is mediated by archaea and the involved processes and carbon flow are a focus of our research activitites.
We use low-molecular weight compounds as “biomarkers” that provide information about substrates, metabolic processes, and syntrophic relationships in microbial ecosystems.

Dissolved organic matter (DOM)
We are interested in dissolved organic matter (DOM) as an important link in the transformation of macromolecular organic matter to low-molecular-weight compounds which serve as substrates for microorganisms such as methanogens and sulfate-reducers. Important insights into the mechanisms of microbially mediated degradation of complex organic matter to terminal substrates can be obtained through molecular analysis of the pool of DOM by ultra-high resolution mass spectrometry (FT-ICR-MS) (Schmidt et al., 2009; 2011). This technique enables simultaneous detection of ~5000 compounds and determination of their elemental composition in a typical sample of marine pore water.
Constraining the alteration of dissolved organic matter on a chemical level will be key to understanding the limitations of subsurface microbial activity and its role in the geological carbon cycle.

Stable carbon isotopes
Stable carbon isotopic investigations are extremely helpful to elucidate carbon flow. Carbon, the main building block of organic compounds, has two stable isotopes. In nature, the heavier carbon-13 isotope is much less abundant than the lighter carbon-12 isotope which accounts for 98.9% of all carbon. However, the exact ratio of 13C and 12C atoms, expressed as δ13C-value, is not constant. In organic compounds it varies because organisms prefer to use the lighter 12C isotope in metabolic processes. The extent of the isotopic fractionation that is eventually reached between substrates and products is characteristic for the involved processes. For instance, autotrophic fixation of CO2 goes along with a strong carbon isotope effect but heterotrophic consumption of large organic molecules doesn't. In this manner, the natural stable isotopic composition of organic metabolites and cell components encodes information on the flow of carbon in ecosystems.
Since 13C is rare in nature, stable isotope probing (SIP), i.e. the addition of 13C-labelled compounds in incubation experimetns, is a very sensitive method to track the flow of carbon from substrates to metabolic products and new biomass.
In order to determine the carbon isotopic composition of a sample, it needs to be oxidized to CO2 first. This approach is most straightforward for the analysis of bulk samples which can be combusted in an elemental analyzer coupled to an isotope-ratio mass spectrometer. In order to obtain δ13C values of individual organic compounds, however, chromatographic seperation needs to preceed their oxidation to CO2 and isotope analysis. This requires online coupling of chromatography and mass spectrometry via a combustion or oxidation interface, also known as isotope ratio monitoring mass spectrometry. While compound specific isotope analysis (CSIA) by isotope-ratio-monitoring gas chromatography/mass spectrometry started more than twenty years ago (Hayes et al., 1990), the carbon isotopic analysis of water-soluble organic compounds by online-coupling of liquid chromatography and mass spectrometry is a rather new technique (Krummen et al., 2004, Heuer et al., 2006). All three techniques are available in the Hinrichs Lab.
Since 13C is rare in nature, stable isotope probing (SIP), i.e. the addition of 13C-labelled compounds in incubation experimetns, is a very sensitive method to track the flow of carbon from substrates to metabolic products and new biomass.
In order to determine the carbon isotopic composition of a sample, it needs to be oxidized to CO2 first. This approach is most straightforward for the analysis of bulk samples which can be combusted in an elemental analyzer coupled to an isotope-ratio mass spectrometer. In order to obtain δ13C values of individual organic compounds, however, chromatographic seperation needs to preceed their oxidation to CO2 and isotope analysis. This requires online coupling of chromatography and mass spectrometry via a combustion or oxidation interface, also known as isotope ratio monitoring mass spectrometry. While compound specific isotope analysis (CSIA) by isotope-ratio-monitoring gas chromatography/mass spectrometry started more than twenty years ago (Hayes et al., 1990), the carbon isotopic analysis of water-soluble organic compounds by online-coupling of liquid chromatography and mass spectrometry is a rather new technique (Krummen et al., 2004, Heuer et al., 2006). All three techniques are available in the Hinrichs Lab.
We constantly seek to extend the range of compounds that can be assesed by compound specific isotope analysis.


