Page path:
- General/Marine Geology
- Gas Hydrate Research
- General Information on Gas Hydrates
General Information on Gas Hydrates
If a mixture of gas and water is exposed to high pressure and low temperature, it turns into a solid, ice-like compound, called gas hydrate. As early as 1810, the British scientist Sir Humphrey Davy happened to produce such a substance (chlorine hydrate) when he let chlorine gas effervesce through water under elevated pressure. However, for more than a century afterwards, gas hydrate was perceived as nothing more than a chemical oddity, and as such it did not receive much attention. In the 1930s, however, the oil and gas industry found out that unexpected formation of gas hydrate clogged pipelines. At low temperatures, gas (predominantly methane) exposed to elevated pressure formed solid gas hydrate.
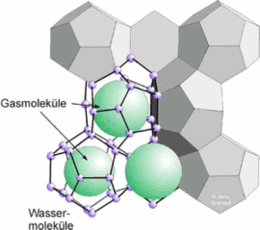
Picture:
Scheme of gas hydrate structure I.
Based upon theoretical models, Russian scientists postulated in the 1970s that there must be natural deposits of gas hydrate. This theory was confirmed in the 1980s when seafloor samples were recovered in the Black Sea from Russian vessels and off Central America by use of the deep-sea drilling ship GLOMAR CHALLENGER. Important issues raised in connection with gas hydrate are:
- a potential use of gas hydrate as a source of methane and as such as energy,
- the interaction of gas hydrate and climate,
- the role of gas hydrate in the global carbon cycle,
- gas hydrate as a factor in the stability of sediments on continental slopes.
These essential, global issues, as well as applied problems such as the stability of oil and gas platforms built on sediments that contain gas hydrate, induced many countries to set up national research programs for the investigation of gas hydrate. The most important ones are run by Japan, Canada, the USA, Germany and India.
- a potential use of gas hydrate as a source of methane and as such as energy,
- the interaction of gas hydrate and climate,
- the role of gas hydrate in the global carbon cycle,
- gas hydrate as a factor in the stability of sediments on continental slopes.
These essential, global issues, as well as applied problems such as the stability of oil and gas platforms built on sediments that contain gas hydrate, induced many countries to set up national research programs for the investigation of gas hydrate. The most important ones are run by Japan, Canada, the USA, Germany and India.


